Abstract
Background and Objective:Ruxolitnib, an orally bioavailable non-specific JAK inhibitor is a novel drug used for treating myelofibrosis by targeting the unique disease biology and has significantly reduced symptomatic burden in these patients. In resource limited countries, the data on post marketing experience with this relatively novel agent is limited. The major objectives of this study was to examine the challenges encountered with the use of Ruxolitinib in our resource limited setting.
Methods:Patients with confirmed diagnosis of myelofibrosis and initiated on ruxolitinib therapy consecutively during the time period between December 2013 to March 2019 at our institute were included in the study. The data was collected from electronic medical records over a period of 12 months from March 2020 to March 2021.
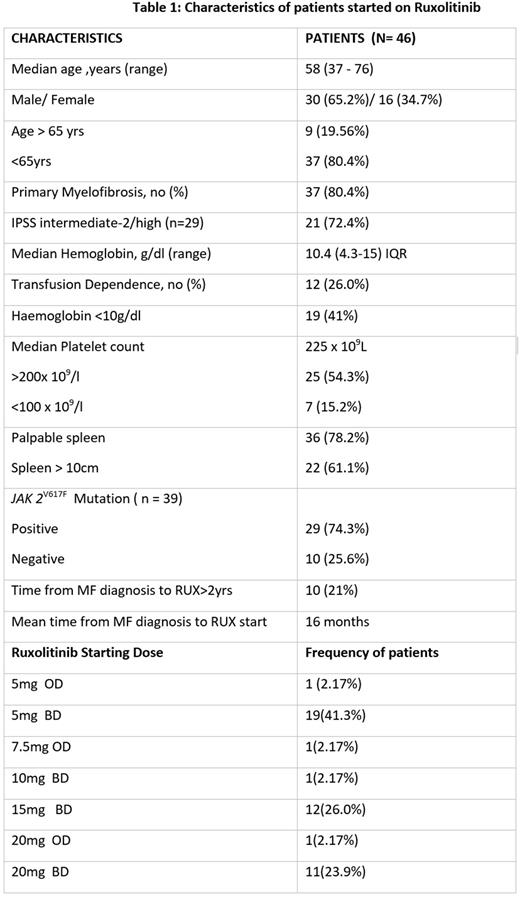
Results:This retrospective analysis included forty six patients and their baseline characteristics are summarized in Table 1. Twenty five (54.3%) patients received lower than the recommended dose for the corresponding platelet count. On response assessment, the mean MPN SAF ISS score showed reduction from 30 (6-64) to 15 (2-38).Among the 36 patients with splenomegaly documented in records, a spleen response below costal margin of <5cm was seen in 2 (5.5%), 5 - 10cm in 9 (25%) and >10cm in 22 (61.1%) patients. . The patients were followed up for a median of 29.5 months (0-76 months) after Ruxolitinib initiation.At the time of data locking, 12 (26%) patients were still on ruxolitinib, 13 (28.2%) patients were lost to follow up and 7 (15.2%) patients died. Rest of the fourteen (30.4%) patients discontinued ruxolitinib, the reasons being financial constraints in 7 (50%), disease progression in 3 (21.4%), haematological toxicity in 2 (14.2%) and intolerance due to non-haematological causes in 2 (14.2%). Haematological adverse events that necessitated dose reduction or cessation of drug were anemia and thrombocytopenia. The serious non-hematological adverse events were renal dysfunction, pancreatitis, COVID infection, DIC and pulmonary alveolar haemorrhage in one patient each and two cases of active tuberculosis. Seven (15.2%) patients died while on therapy or shortly after discontinuing ruxolitinib. The causes of death were pulmonary alveolar haemorrhage, disease progression, disseminated intravascular coagulation post cardiac valve surgery, pneumonia complicated by sepsis/ hemophagocytic lymphohistiocytosis (HLH) and COVID infection. Among these, 2 patients died within one month of stopping ruxolitinib. The possibility of ruxolitinib discontinuation syndrome (RDS) was considered retrospectively in these patients. The overall mean survival time was 145.4 ±16 months (95% CI: 114 - 176.8months).
Discussion:At the time of last follow-up, more than half of the patients in our study group had either discontinued therapy or were lost to follow up. The most common reason for discontinuation was financial burden, the other reasons being disease progression and intolerance. In our setting, the patients entail an annual cost of approximately 2560 USD, 4530 USD and 4770 USD for 5mg BD, 15mg BD and 20mg BD respectively within patient assisted programs and much higher outside these programs. In a country like India with a nominal Gross Domestic Product (GDP) per capita of around 2191 USD, this not only reflects the high cost of the drug but also the discordant pricing of the different doses resulting in opting for lower doses. The financial burden of the drug is also reflected in the NICE Appraisal Committee recommendation that though ruxolitinib is clinically effective, it is not cost effective for symptomatic therapy CONCLUSION
In spite of having a good symptom response, many of our patients discontinued the drug because of its "financial toxicity". In developing economies, better access to the drug and doing away with suboptimal doses need to be addressed so that the target population can reap its therapeutic effect in entirety.
Disclosures
Sidharthan:Astra Zeneca: Speakers Bureau; Emcure: Consultancy; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; jansen: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal